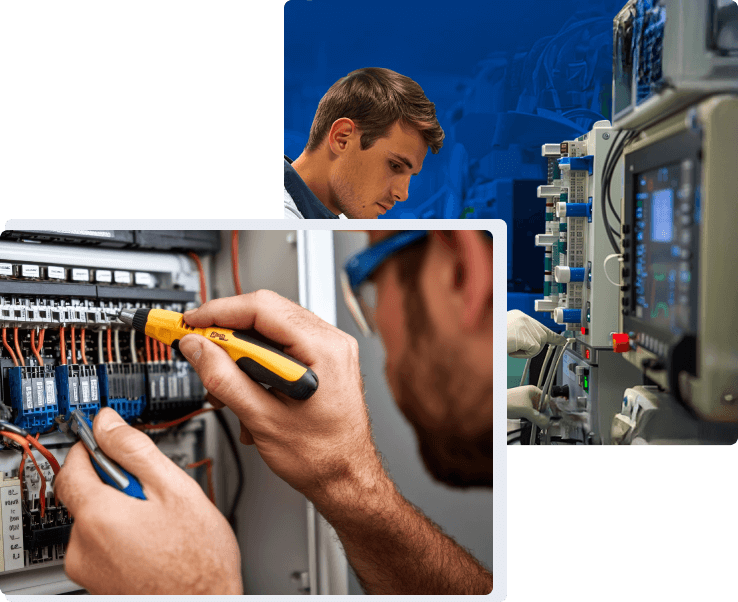
Raising the Standard for Medical Device Safety
Our expert teams support manufacturers through rigorous evaluations of electrical, mechanical, and environmental safety to ensure devices meet global regulatory expectations. With FDA ASCA-accredited labs and decades of medical testing expertise, Eurofins Electrical & Electronics helps bring your innovations to market with speed, confidence, and integrity. Every test we perform safeguards not just compliance, but medical professionals and their patients.

.png?width=122&height=104&name=Layer_1%20(1).png)






.png)
.png?width=146&height=146&name=Mask%20group%20(3).png)
.png?width=146&height=146&name=Mask%20group%20(4).png)
.png?width=146&height=146&name=Mask%20group%20(5).png)
.png?width=146&height=146&name=Mask%20group%20(6).png)
.png?width=146&height=146&name=Mask%20group%20(7).png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)
.png)

-1.png)
-1.png)
-1.png)
-1.png)
-1.png)
-1.png)
-1.png)
-1.png)
